In the life sciences industry, where regulatory compliance is non-negotiable, having a well-structured compliance training program is essential. These programs not only ensure that employees understand the regulations governing their work but also protect the organization from potential legal and financial repercussions. To ensure success, it is crucial to follow best practices for compliance training. Here are some key best practices for designing and implementing effective compliance training programs tailored for life sciences companies.
What is Compliance Training?
Compliance training refers to the process of educating employees about the laws, regulations, and company policies that are relevant to their job roles and industry. This type of training ensures that employees understand their responsibilities and adhere to both legal standards and organizational values. By conducting regular compliance training, businesses can mitigate risks, avoid penalties, and maintain a culture of ethical behavior.
Popular topics covered in compliance training include:
- Data security and privacy laws
- Workplace harassment prevention
- Anti-bribery and corruption regulations
- Environmental compliance
- Health and safety guidelines
Effective compliance training leverages tools like Learning Management Systems (LMS) to deliver consistent, trackable, and engaging training modules. These systems also allow organizations to customize training content based on specific regulatory needs.
Why is Compliance Training Important?
Compliance training helps organizations:
- Reduce the risk of legal and financial repercussions.
- Enhance employee knowledge and accountability.
- Protect company reputation.
- Ensure adherence to industry-specific regulations like FDA guidelines or OSHA standards.
What is Life Science Compliance Training?
Life science compliance training focuses specifically on the regulations and standards governing the life sciences industry, such as pharmaceuticals, biotechnology, and medical devices. This specialized training ensures that employees in these sectors understand and comply with critical guidelines, including:
- FDA regulations (e.g., 21 CFR Part 11)
- Good Manufacturing Practices (GMP)
- Good Clinical Practices (GCP)
- Data integrity and patient safety standards
Life science compliance training is crucial for maintaining operational integrity, ensuring product quality, and avoiding regulatory violations. Programs often incorporate examples, case studies, and interactive scenarios to address the unique challenges of the life sciences sector.
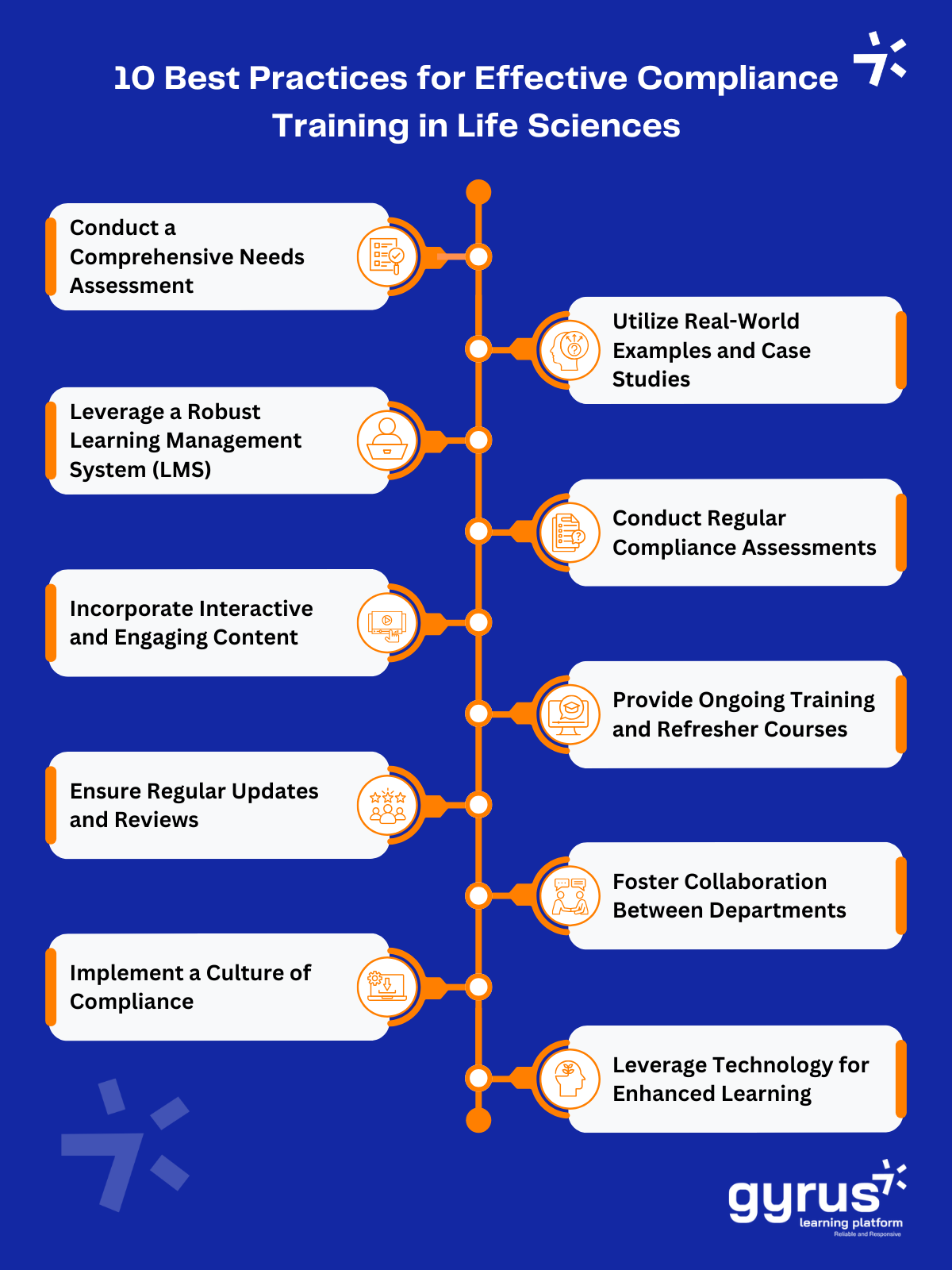
10 Essential Best Practices for Effective Compliance Training in the Life Sciences
1. Conduct a Comprehensive Needs Assessment
Before launching a compliance training program, organizations should conduct a thorough needs assessment to identify the specific training requirements based on their operations, regulatory environment, and employee roles. This assessment should consider:
- Applicable Regulations: Understand the regulations relevant to your industry, such as FDA guidelines, 21 CFR Part 11, and Good Manufacturing Practices (GMP).
- Employee Roles: Different roles within the organization may require distinct training approaches. Tailor training content to meet the unique needs of different employee groups.
By pinpointing these factors, organizations can develop targeted training that addresses compliance effectively.
2. Leverage a Robust Learning Management System (LMS)
Implementing a Learning Management System (LMS) is crucial for streamlining compliance training. A robust LMS allows organizations to:
- Track Training Progress: Monitor employee participation and completion rates to ensure compliance training is effectively reaching all team members.
- Store Training Materials: Centralize all training resources in one location, making it easy for employees to access the information they need.
GyrusAim LMS offers features specifically designed for compliance tracking, ensuring that organizations can efficiently manage their training initiatives.
3. Incorporate Interactive and Engaging Content
To enhance retention and engagement, compliance training should incorporate a variety of interactive and engaging content formats. This could include:
- E-learning Modules: Develop online courses that employees can complete at their own pace, providing flexibility and convenience.
- Simulations and Scenarios: Use realistic scenarios and simulations to help employees practice applying compliance concepts in real-life situations.
- Quizzes and Assessments: Incorporate regular quizzes to reinforce learning and assess understanding.
By diversifying content delivery, organizations can maintain employee interest and improve knowledge retention.
4. Ensure Regular Updates and Reviews
The life sciences industry is constantly evolving, with regulations frequently updated to reflect new scientific knowledge and societal needs. Organizations should establish a process for regularly reviewing and updating training materials to ensure they remain relevant and compliant. This could include following best practices for compliance training, such as:
- Scheduled Reviews: Set up a schedule for regular content reviews, ensuring that training materials align with the latest regulations and best practices.
- Feedback Mechanisms: Gather employee feedback on training materials and effectiveness, using this input to make necessary adjustments.
Keeping training materials up-to-date is vital for maintaining compliance and ensuring employees are informed about current standards.
5. Implement a Culture of Compliance
Creating a culture of compliance within the organization is essential for fostering accountability and ethical behavior among employees. To achieve this, organizations should:
- Lead by Example: Leadership should actively participate in compliance training and demonstrate a commitment to ethical practices.
- Encourage Open Communication: Foster an environment where employees feel comfortable discussing compliance issues or reporting concerns without fear of retaliation.
By embedding compliance into the company culture, organizations can enhance the effectiveness of their training programs and encourage responsible behavior.
6. Utilize Real-World Examples and Case Studies
Incorporating real-world examples and case studies into training can help employees understand the practical implications of compliance. Highlighting instances of regulatory breaches and their consequences can emphasize the importance of adhering to compliance standards. This approach not only reinforces the seriousness of compliance but also provides valuable learning opportunities.
7. Conduct Regular Compliance Assessments
Organizations should conduct regular assessments to evaluate the effectiveness of their compliance training programs. These assessments can include:
- Surveys and Feedback: Gather employee feedback on the training process, content, and relevance to their roles.
- Knowledge Checks: Regularly assess employees’ understanding of compliance topics through quizzes and tests.
By continually assessing training effectiveness, organizations can identify areas for improvement and ensure their programs meet compliance needs.
8. Provide Ongoing Training and Refresher Courses
Compliance training should not be a one-time event. Regular refresher courses and ongoing training opportunities are crucial for reinforcing knowledge and ensuring employees remain up-to-date with compliance requirements. This can include:
- Annual Training Updates: Implement mandatory annual compliance training to review key concepts and updates.
- On-Demand Learning Resources: Provide access to a library of compliance-related resources that employees can reference as needed.
Ongoing training helps employees stay informed and prepared to navigate the complexities of compliance.
9. Foster Collaboration Between Departments
Compliance training is not limited to a single department; it affects all aspects of an organization. Encourage collaboration between departments, such as quality assurance, regulatory affairs, and human resources, to develop comprehensive training programs that address the needs of all teams.
By involving multiple stakeholders, organizations can ensure that compliance training is holistic and covers all relevant aspects of operations.
10. Leverage Technology for Enhanced Learning
Advancements in technology can greatly enhance compliance training programs. Organizations should consider incorporating innovative technologies such as:
- Virtual Reality (VR) Training: Utilize VR simulations to immerse employees in realistic training scenarios.
- Mobile Learning Solutions: Offer mobile-friendly training options to allow employees to complete training on-the-go.
By leveraging technology, organizations can create engaging and effective training experiences that resonate with employees. Click here to learn more about top learning technologies you can’t overlook.
What is the Purpose of Ethics and Compliance Training?
Ethics and compliance training serves as the foundation for fostering a responsible and ethical workplace culture while ensuring adherence to regulatory requirements. In the life sciences industry, this training is critical for:
- Understanding Regulatory Requirements: Employees gain a clear understanding of laws, regulations, and industry standards such as FDA guidelines, 21 CFR Part 11, and GMP, which govern their daily operations.
- Reducing Risks: By educating employees on compliance standards, organizations minimize the risk of regulatory violations, legal consequences, and reputational damage.
- Promoting Ethical Behavior: Ethics training goes beyond compliance by embedding values that encourage accountability, transparency, and ethical decision-making.
- Enhancing Operational Efficiency: A well-informed workforce reduces errors, ensures consistent processes, and strengthens quality assurance across the organization.
Ethics and compliance training is more than a checkbox; it’s a proactive approach to building trust with stakeholders and maintaining a strong reputation in a highly regulated industry.
What do Regulators Expect of your Life Science Compliance Training Program?
Regulators demand life sciences compliance training programs to be robust, comprehensive, and continuously updated to meet evolving standards. Specific expectations include:
- Tailored Content: Training must address the specific roles, responsibilities, and risks associated with each position in the organization.
- Clear Understanding of Regulations: Programs should cover all relevant regulatory frameworks, such as FDA guidelines, HIPAA, GMP, and 21 CFR Part 11, ensuring employees understand how they apply to their work.
- Demonstrated Effectiveness: Organizations must show evidence that employees retain the knowledge and can apply it effectively. This is often done through assessments, quizzes, and real-world scenario training.
- Documented Compliance: Regulators expect detailed records of training attendance, completion rates, and content updates. A robust LMS can simplify this process by maintaining an audit trail.
- Regular Updates: Training must reflect changes in regulatory requirements, industry best practices, and technological advancements.
Meeting these expectations not only satisfies regulators but also fosters a culture of compliance, reducing the likelihood of costly penalties.
Life Science Compliance Training Course Examples
To meet diverse regulatory requirements and operational challenges, life sciences companies should offer specialized compliance training courses. Examples include:
- 21 CFR Part 11 Compliance: Training on electronic records and signatures to ensure adherence to FDA requirements.
- Good Manufacturing Practices (GMP): Detailed guidance on maintaining quality standards in manufacturing processes.
- HIPAA Compliance: Focused training on protecting patient data and ensuring data privacy in clinical trials or healthcare-related operations.
- Anti-Bribery and Corruption: Educating employees on identifying and avoiding unethical practices in global operations.
- Data Integrity and Cybersecurity: Courses on safeguarding research data, preventing breaches, and ensuring secure data management.
- Workplace Safety and Hazardous Materials Handling: Training employees on safe practices for handling biological samples, chemicals, or other hazardous materials.
These courses should be interactive, role-specific, and updated regularly to reflect the latest regulatory requirements.
How Should Your Compliance Training Program be Customized?
A one-size-fits-all approach is ineffective for compliance training in the life sciences industry. Customization ensures that training is relevant, engaging, and impactful. Consider the following strategies:
- Role-Specific Training: Different roles require different compliance knowledge. Customize training for manufacturing staff, clinical teams, sales representatives, and leadership.
- Regional Regulations: Adapt content to reflect regulatory differences in regions where the organization operates, such as FDA in the U.S. or EMA in Europe.
- Interactive Content Formats: Use simulations, case studies, and scenario-based training to make compliance concepts relatable to real-life situations.
- Integration with Company Policies: Align training materials with the organization’s specific policies, procedures, and ethical standards.
- Flexible Delivery Methods: Offer a mix of e-learning, in-person workshops, and mobile-friendly training options to cater to diverse learning preferences.
Customization enhances the relevance and effectiveness of compliance training, ensuring employees can confidently apply their knowledge in their roles.
Examples of Compliance Training Programs
Here are examples of compliance training programs that effectively address critical areas in the life sciences industry:
- FDA Inspection Readiness Program: Prepares employees to handle FDA inspections confidently by understanding documentation, procedures, and best practices.
- Data Privacy and GDPR Compliance Training: Focuses on managing patient and clinical data in compliance with privacy laws like GDPR and HIPAA.
- Clinical Trial Compliance Training: Provides guidelines on conducting trials ethically and in adherence to regulatory standards.
- Supply Chain and Vendor Compliance Training: Teaches teams to evaluate and ensure that vendors meet required compliance standards.
- Environmental Health and Safety (EHS) Training: Ensures employees are trained in environmental compliance, workplace safety, and hazardous waste management.
- Quality Control and Risk Management: Educates employees on maintaining high-quality standards and implementing risk management protocols.
These programs, tailored to specific regulatory and operational requirements, are integral to maintaining compliance and operational integrity in the life sciences industry.
Conclusion
Implementing effective compliance training in the life sciences industry is crucial for ensuring regulatory adherence and maintaining operational integrity. By following these best practices for compliance training, organizations can develop training programs that not only meet compliance requirements but also foster a culture of continuous learning and improvement.
Investing in a robust LMS, such as GyrusAim, can facilitate the development and management of compliance training programs, empowering employees with the knowledge and skills they need to excel in their roles. As the life sciences landscape continues to evolve, following the best practices for compliance training will remain a cornerstone of operational success.
Common Questions About Life Science Compliance Training
1. What are the 4 C’s of compliance?
The 4 C’s of compliance are Culture, Commitment, Control, and Communication. These elements help ensure that compliance is integrated into the organization’s culture, maintained through strong commitment, supported by effective controls, and communicated clearly throughout the organization.
2. What are the 4 Ps of compliance?
The 4 Ps of compliance are Policies, Processes, Procedures, and Performance. These are key components for ensuring an organization’s compliance with regulatory standards and maintaining high operational integrity.
3. What are the 3 P’s of compliance?
The 3 P’s of compliance are Prevention, Detection, and Correction. This framework helps organizations prevent compliance violations, detect any issues early, and correct them promptly to maintain compliance with industry regulations.
4. What are the 7 pillars of compliance?
The 7 pillars of compliance are Governance, Risk Management, Internal Controls, Audit and Monitoring, Training and Awareness, Reporting, and Enforcement. These elements ensure a robust compliance program that aligns with legal requirements and industry best practices.
5. What are the 3 main pillars of compliance?
The 3 main pillars of compliance are Prevention, Detection, and Response. A strong compliance program focuses on preventing violations, detecting any issues early, and responding effectively to maintain compliance.
6. What makes good compliance training?
Good compliance training is interactive, engaging, and tailored to your industry’s specific regulatory needs, such as life sciences compliance. It ensures that employees understand the rules, can apply them in their work, and are motivated to stay compliant through continuous education.
7. How to make compliance training fun?
To make compliance training fun, incorporate interactive elements like gamification, real-life scenarios, and quizzes. Using engaging compliance training methods helps employees stay motivated while learning essential compliance topics like regulatory updates and ethical practices.
8. What are the elements of compliance training?
The key elements of compliance training include clear objectives, engaging content, regular assessments, and ongoing updates. Effective compliance training also ensures employees understand legal requirements, industry-specific standards, and best practices for maintaining compliance.
9. What are the three compliance techniques?
The three compliance techniques are monitoring, auditing, and training. Regular monitoring helps identify potential issues, audits ensure compliance with regulations, and continuous training reinforces knowledge and best practices.
10. What is an effective compliance program?
An effective compliance program includes clear policies, regular employee training, strong internal controls, and mechanisms for reporting and addressing violations. It should be tailored to the industry’s specific regulations, like life sciences compliance, to ensure full compliance with all laws and standards.
11. What is the first line of compliance?
The first line of compliance is the management and employees who are directly responsible for adhering to policies and procedures. They play a crucial role in maintaining a culture of compliance by following established guidelines and reporting any potential violations.